How hydrogen is produced
One element, different ways to produce it

Hydrogen is the most abundant element in the universe, however on our planet we only find it bound to other elements, such as oxygen or carbon. In fact, it is called an energy vector, and not a source, as we must use energy to separate and extract it, and this kind of process has costs.
All the colors of hydrogen
Hydrogen has different names depending on how it is produced, and the resulting environmental impact of the processes used to obtain it.
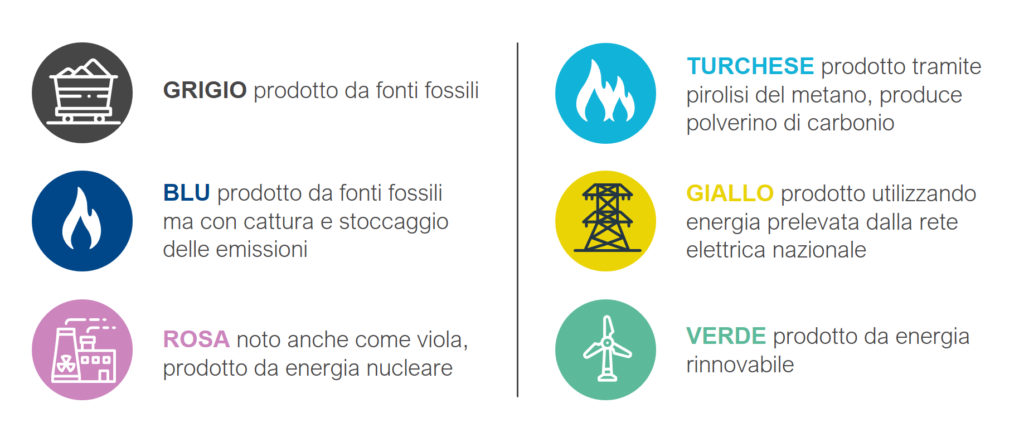
Picture “The colors of hydrogen” (in Italian)
Grey hydrogen: it is produced from fossil sources
Blue hydrogen: it is produced from fossil sources but captured and stored
Rose hydrogen: also known as purple, it is produced from nuclear energy
Turquoise hydrogen: it is produced by pyrolysis of methane, it produces carbon char
Yellow hydrogen: it is produced using energy drawn from the national power grid
Green hydrogen: it is produced from renewable energy
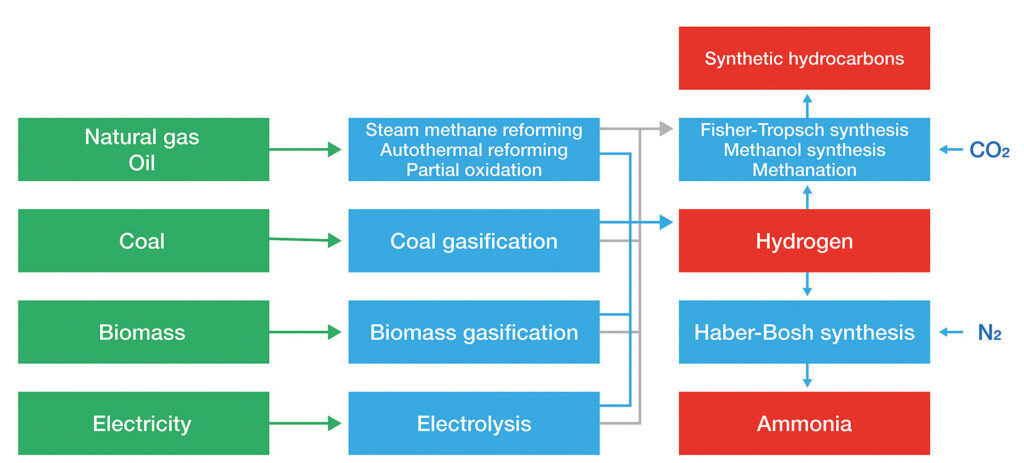
Summary of the different methods of hydrogen production
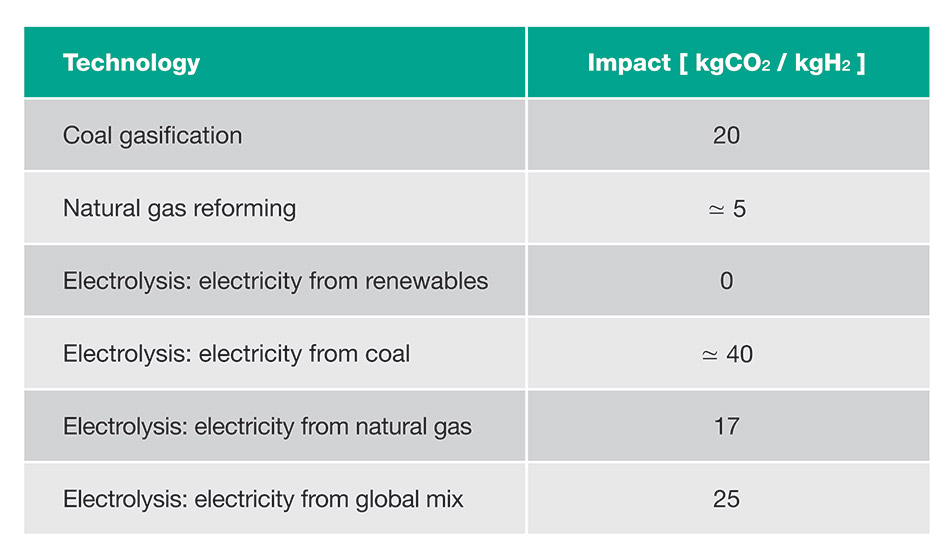
CO2 emissions based on the hydrogen production technology used
One of the main methods by which hydrogen generation can take place is by electrolysis, a process known as early as the nineteenth century, in which the passage of electric current through water causes it to split into oxygen and hydrogen gases.
Hydrogen produced using renewable energy sources is called green hydrogen, which is currently produced through two main technologies: alkaline (AEC), and proton exchange membrane (PEM).
Some studies are now focusing on new ways to obtain it by exploiting the activity of red bacteria, cyanobacteria, and microalgae, as well as the gasification of municipal solid waste and non-recyclable plastics, through the decomposition of organic matter with thermal treatments at high temperatures in a nearly oxygen-free atmosphere.
A new technology for hydrogen production
Hyter, a company part of the Pietro Fiorentini Group since 2021, has developed a hydrogen generator based on innovative AEMWE, Anion Exchange Membrane Water Electrolyser, technology.

The electrolyser supplied by Hyter combines the advantages of both PEM and alkaline systems, allowing reduced investment costs and lower environmental impact thanks to reduced use of noble metals.
Ion exchange takes place through a polymer membrane produced in-house by Hyter. This membrane contains electrolytic substances that improve the efficiency of water splitting and has the function of separating hydrogen and oxygen already during the production phase.
This technology is much less expensive chemically and at the same time possesses excellent efficiency in terms of yield.
The electrolyser supplied by Hyter will be used within a project for PLT Energia, a company that has been presiding over the entire value chain of renewables for 15 years. The new Hyter electrolyser will interface with the photovoltaic system operating on the buildings of PLT Energia: in this way, it will be possible to produce the hydrogen needed to guarantee the environmental sustainability of the electrical and thermal consumption of their headquarters.
A green hydrogen plant for PLT energia thanks to the Hyter electrolyser
Hyter was recently awarded one of MiTE’s calls for tenders with the Sirius project, which involves the construction within three years of the world’s first electrolyser with a size greater than one megawatt based on AEMWE technology, Anion Exchange Membrane Water Electrolyser. The Sirius project, which in addition to Hyter also has the participation of INRETE Distribuzione Energia (Hera Group), MatRes (MBN Nanomaterialia Group) and the Department of Chemical Sciences at the University of Padua, ranked third in the category “Mission 2 – Component 2 – Investment 3. 5 – Hydrogen Research and Development” among the fifty-six proposals submitted to the Italian Ministry of Ecological Transition to receive European Union funding provided under the PNRR, National Economic Recovery and Resilience Plan.
Project financed under the PNRR, co-financed by the European Union – NextGenerationEU.


